This doc describes how to use Boolean search to retrieve records from ClinicalTrials.gov.
To perform a ClinicalTrials.gov search into your nest, you can either 1. Write a query from scratch, or 2. Copy and paste the query from a search in ClinicalTrials.gov. Guidance for both options is given below.
How to write a ClinicalTrials.gov query #
Use AND/OR/NOT, in combination with keywords to narrow in on trials of interest. For example:
dizziness AND NOT exhaustion AND stroke
will retrieve trials with the word “dizziness” and “stroke” contained somewhere in the record: the trial title, description, interventions, conditions, or several other fields; trials with “exhaustion” mentioned anywhere will be excluded from results. Use parentheses if the logical operators become numerous and ambiguous, and use quotes if your keywords contain spaces.
To generate a more precise search than the Basic Search outlined above, use AREA to search specific fields. If you wanted to limit your search to only trials with these keywords as Conditions being studied:
AREA[ConditionSearch]dizziness AND NOT AREA[ConditionSearch]exhaustion AND AREA[ConditionSearch]stroke
The syntax for AREA searches is simply AREA[area name]search. Common area names include:
- BasicSearch: This is the default, when no AREA is specified. It searches all of the common fields within trials
- ConditionSearch: Searches indexed conditions, condition MeSH terms, the title, and keywords
- InterventionSearch: Searches indexed interventions, intervention MeSH terms, arm labels, keywords, and more
- OutcomeSearch: Searches all outcome fields registered (Primary, Secondary, and Other)
- TitleSearch: Searches the trial name & acronym
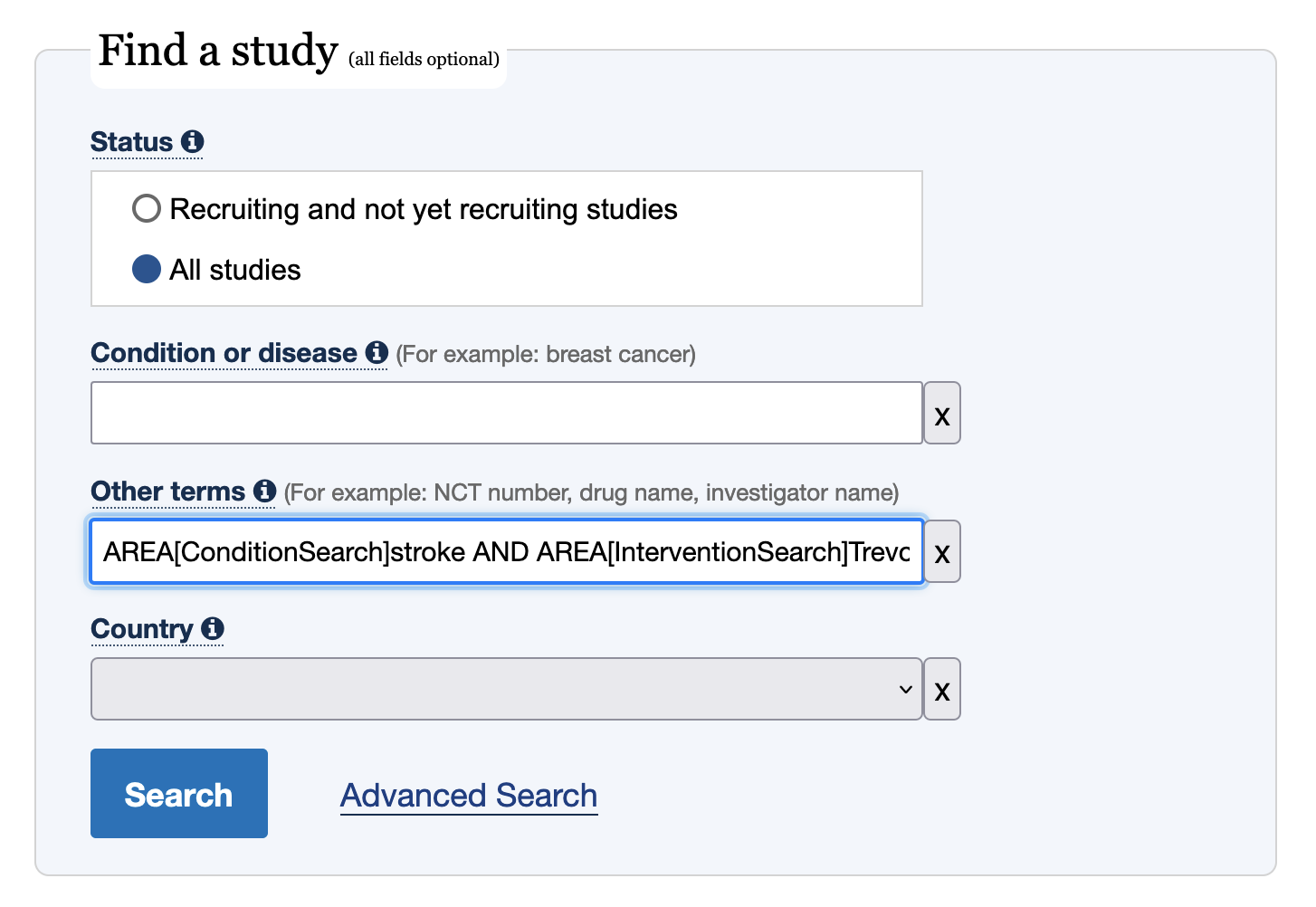
In action, here’s a search for trials assessing mortality of stroke patients treated with the Trevo device:
AREA[ConditionSearch]stroke AND AREA[InterventionSearch]Trevo AND AREA[OutcomeSearch]mortality
Testing your Search Before Running #
You may wish to inspect the quality or volume of results retrieved prior to running an API Import on Nested Knowledge. To test your search, copy paste the search string into https://www.clinicaltrials.gov in the “Other Terms” text input. Note that if you are using “Classic ClinicalTrials.gov” (will be going offline in 2024), results will be different (+/- 1% in our experience), due to searching differences between the versions. Nested Knowledge API integration uses the newer version.
AREAs #
ClinicalTrials.gov publishes a full list of AREAs that may be searched & which fields are included in that AREA here. While BasicSearch, ConditionSearch, InterventionSearch, and OutcomeSearch are most common, field-level AREAs like DesignAllocation and OverallStatus may be useful for certain review types or protocols. For example, to retrieve completed randomized trials of the Trevo device:
AREA[DesignAllocation]Randomized AND AREA[OverallStatus]Completed AND AREA[InterventionSearch]Trevo
Operators #
A full listing of operators may be found here. While AND/OR/NOT/()/“”/AREA are expected to be most common, operators such as RANGE can prove useful. For example, to retrieve the most recent trials on COVID-19:
AREA[ConditionSearch]COVID-19 and AREA[StartDate]RANGE[01/01/2022, MAX]
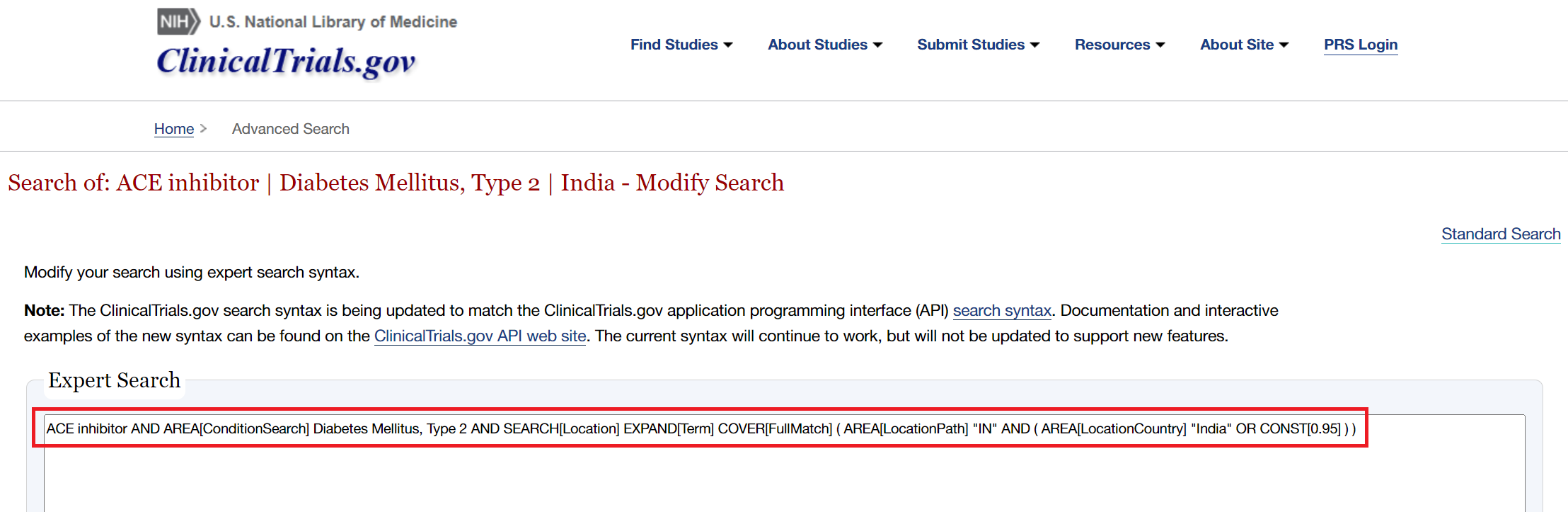
Alternative Option: Copy Query from ClinicalTrials.gov #
If you have already performed a search in ClinicalTrials.gov, and simply want to copy that query into Literature Search so it continues updating on your schedule, follow these steps:
- Input search terms as normal and click “Search”
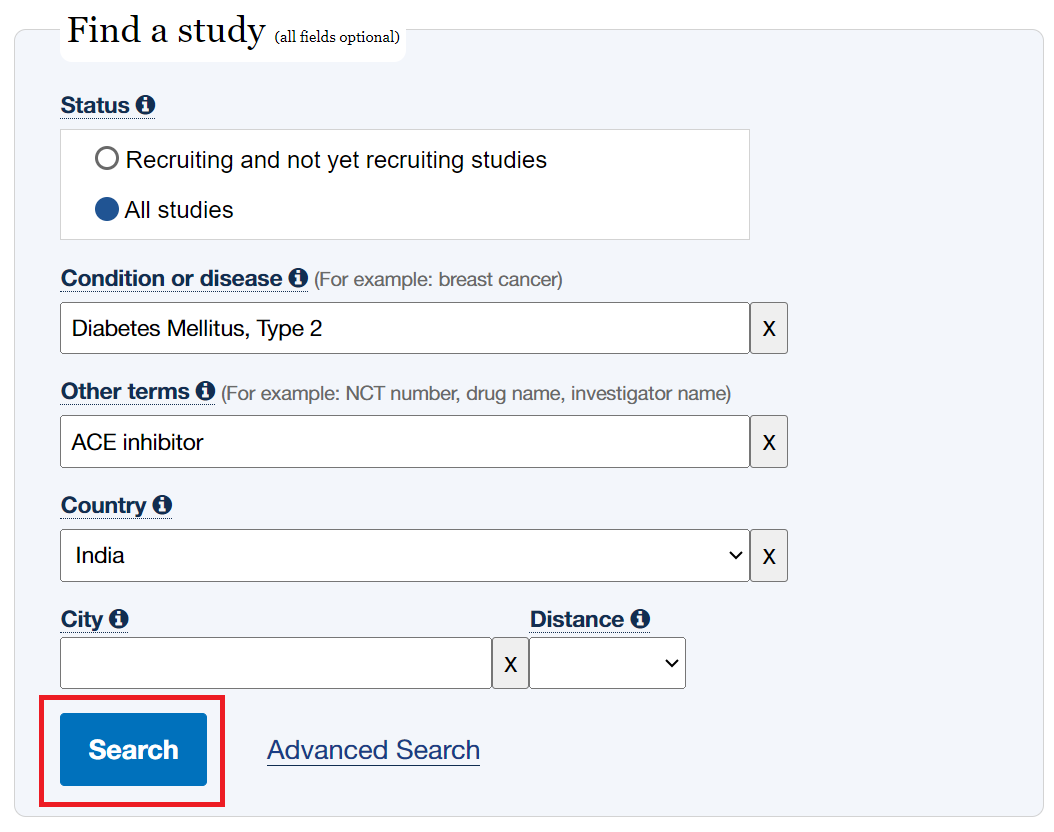
- In the search bars at the top click “Advanced Search”
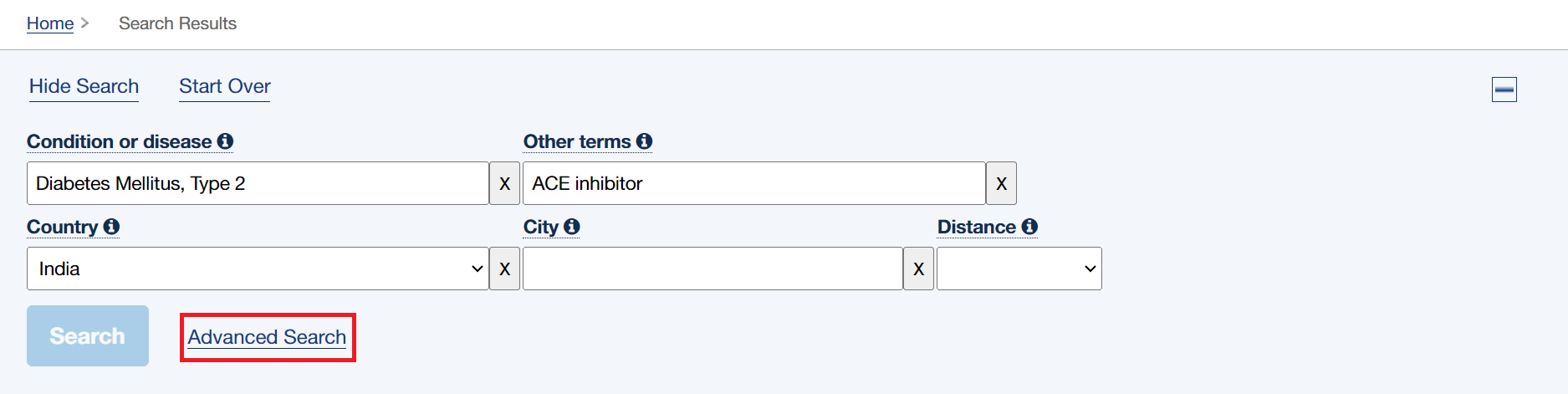
- Then click “Expert Search” in the top right
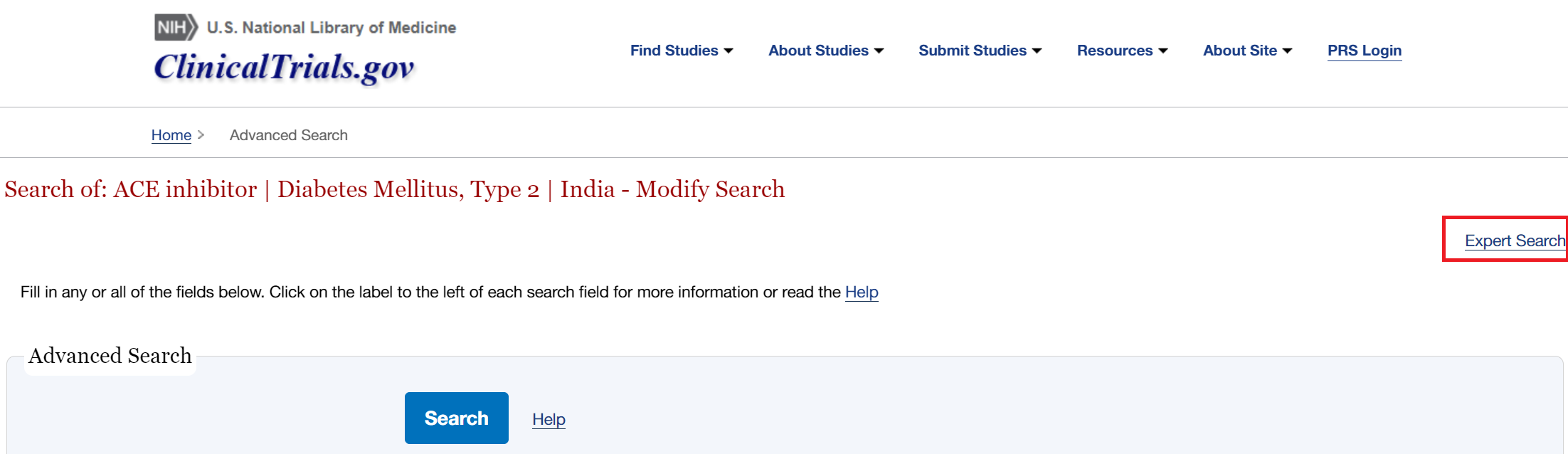
- Your query will then be displayed in the correct format. Copy and paste this into the automatic search in NK.
Integration with NK #
You can conduct searches of clinicaltrials.gov on the Search module of the NK platform. Results from this search will automatically mark papers as Related Reports.

