How Smart Study Type Tags Are Reinventing Evidence Synthesis
One of the features of Core Smart Tags is Smart Study Type – this refers to our AI system that automatically categorises the study type

When a clinical trial evaluates a novel drug or uncovers a change in medical best practices, the results are often published in a widely-read scientific journal, like JAMA, Lancet, or the NEJM. Even trials that don’t unveil the next blockbuster drug are vital. Trials showing no effect compared to placebo, so-called “negative” or inconclusive trials, shape the evidence landscape as much as the splashy headlines.
These trials lie buried in less popular sources: clinical trial registries. While physicians might skim through an issue of JAMA each month, no physician has time to sift through all trial registries!
Instead, systematic reviewers and guideline groups must combine extant medical evidence from trials and condense the results into a publication or recommendation. Thankfully, tools for retrieving trial contents alleviate this burden.
Why Include Clinical Trials?
By failing to include clinical trial records, researchers may overlook data that is critical to their review. Trials reporting no effect and trials terminated early often do not reach journal pages, but many of them are searchable in online trial registries. Excluding “negative” and no-effect findings distorts the evidence. To effectively identify all relevant trials for a systematic review, experts recommend searching the US-maintained ClinicalTrials.gov registry to access key data.
Need for PDFs
A variety of review tools integrate directly with ClinicalTrials.gov, but a workflow problem remains: ClinicalTrials.gov records are not available as PDFs. In a systematic review, researchers and machines alike extract data from PDFs to evaluate therapies and compare outcomes. Pre-prints, books, and scientific articles bounce across the internet as PDFs, but trial registries provide data in other formats, like HTML and JSON.
ClinicalTrials.gov records do not always correspond one-to-one with published results in a journal, and not all trials are published, as publication is a costly, time-intensive process not feasible for all research groups. Some trials include links to related full text publications, but trials frequently have multiple publications–making it unclear which one to include in a review. When a trial has no associated publication, the results must be accessed through a webpage on a trial registry.
While PDFs are the universal medium of scientific communication, ClinicalTrials.gov records cannot be exported as a PDF. For researchers, this means endless clicking, printing, and stitching together web pages just to get a usable document.
How can researchers identify full texts for unpublished clinical trials?
To effectively capture evidence from unpublished trials, systematic-reviewers must include ClinicalTrials.gov reports in their data extraction process. As more researchers adopt AI with oversight, trial data needs to be available in formats understandable and accepted by both Large Language Models and people: PDFs. Nested Knowledge helps researchers surface, organize, and extract clinical trial information in PDFs. Presently, reviewers export clinical trial data by navigating across many trial tabs, printing each tab, and combining the webpages, adding to the tedium that accompanies many systematic reviews.
To achieve parity with published research articles (and to reduce the global burden of combining PDFs), Nested Knowledge’s tool converts ClinicalTrial.gov records to PDFs for you!
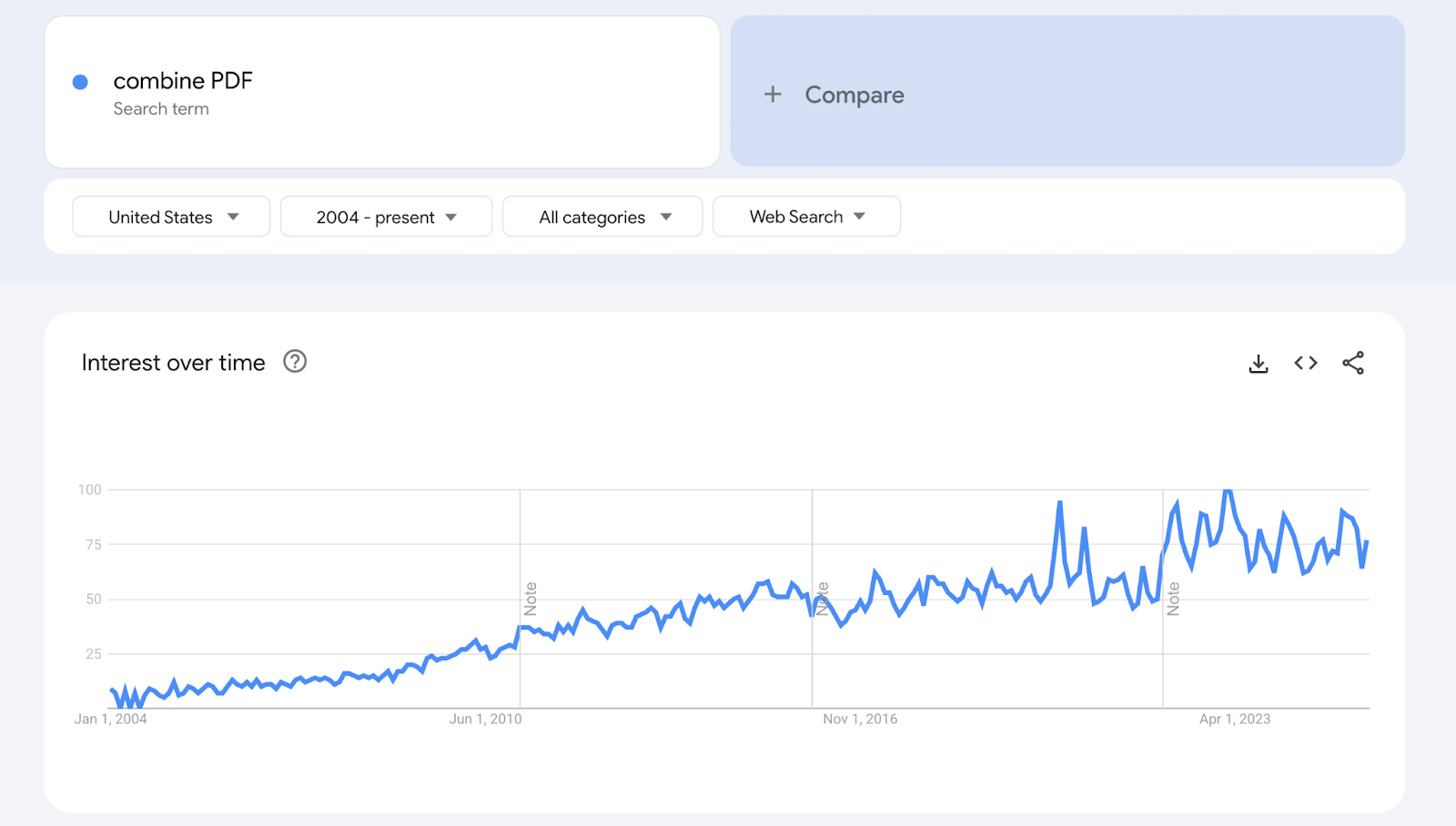
We’re excited to introduce our Clinical Trial report generator, launched in April 2025. With one click, researchers can now turn ClinicalTrials.gov records into clean, review-ready PDFs.
Researchers may then manually extract data from the platform, or use Nested Knowledge’s automated data extraction Adaptive Smart Tags to retrieve data from text and tables. The goal of a review is to capture the methods and outcomes of all relevant studies, and ClinicalTrial.gov helps ensure full coverage and transparent methods. Capturing ClinicalTrial reports is key to performing an unbiased, high-powered review aligned with best practices.
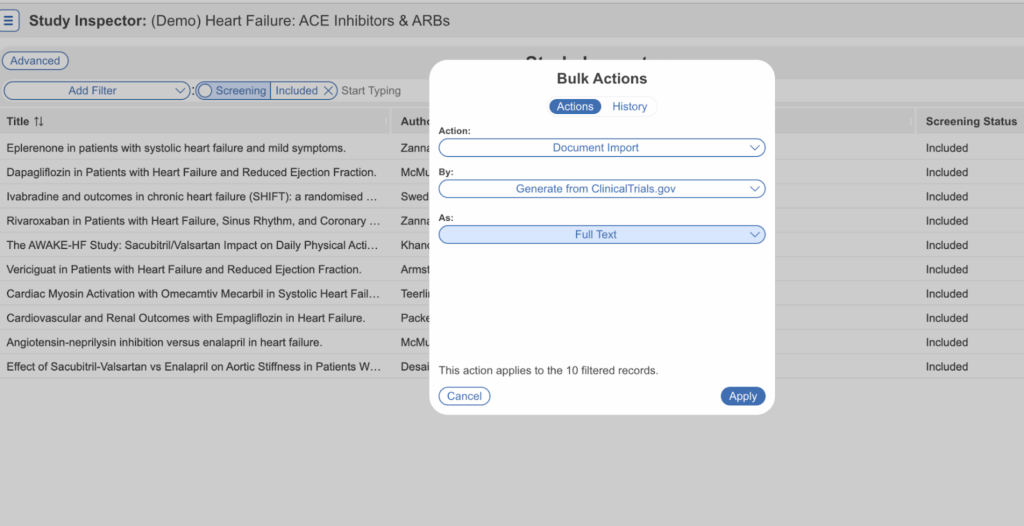
Aggregating data from clinical trials has applications beyond systematic review and meta-analysis. For instance, neurovascular scientists used Nested Knowledge to characterize common data elements reported in trials. With trial data accessible in PDFs, the possibilities multiply! Use cases for Clinical Trial search, report generation, and data extraction include:
To get started in Nested Knowledge, go to https://nested-knowledge.com/sign-up or contact us.
Yep, you read that right. We started making software for conducting systematic reviews because we like doing systematic reviews. And we bet you do too.
If you do, check out this featured post and come back often! We post all the time about best practices, new software features, and upcoming collaborations (that you can join!).
Better yet, subscribe to our blog, and get each new post straight to your inbox.

One of the features of Core Smart Tags is Smart Study Type – this refers to our AI system that automatically categorises the study type