How Smart Study Type Tags Are Reinventing Evidence Synthesis
One of the features of Core Smart Tags is Smart Study Type – this refers to our AI system that automatically categorises the study type

If you say a word enough times it begins to sound like gibberish. Try it: “access, access, access, access, access, access” What does ‘access’ mean anymore? “Lived experience, lived experience, lived experience, lived experience, lived experience.” Does it sound empty yet? New terms ought to be used judiciously. In the field of health economics and outcomes research (HEOR), a new term is budding: patient-centered Health Technology Assessment (HTA). Will patient-centered HTA bloom into a useful technique or wither into a nebulous buzzword?
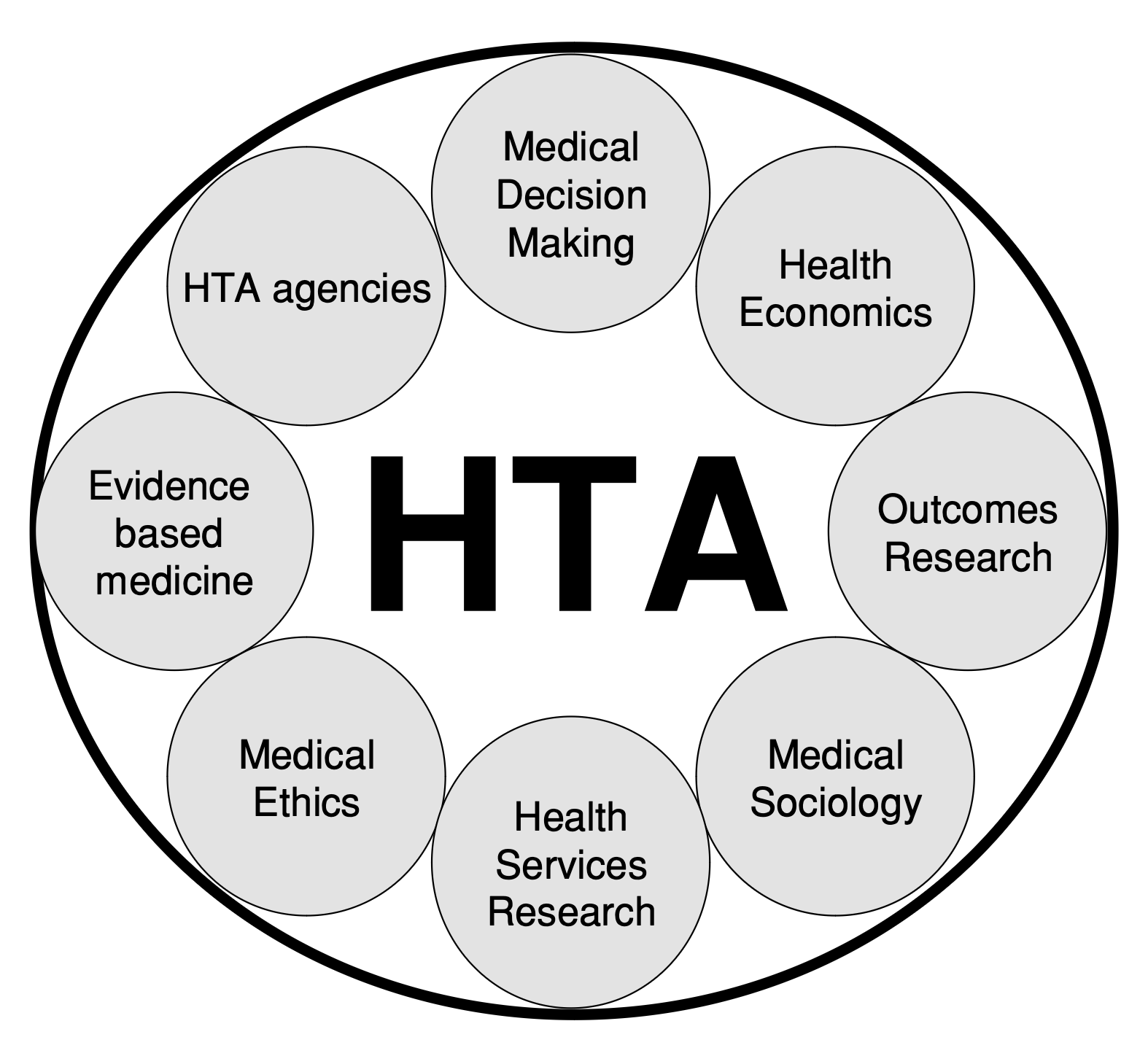
Health Technology Assessment, also called value assessment, refers to the process of estimating the value of drugs and other medical interventions. Clinical evidence, economic modeling, medical ethics, and cost-effectiveness analysis compose HTAs. The results inform which treatments health insurers cover, which in turn affects patients’ access to treatment, which in turn affects the patients’ caregivers, families, dependents, and employers. While HTAs are designed to help payers–in some cases governments– allocate resources and control rising healthcare costs, patients are the ultimate stakeholders.
In the seventeen years since researchers first proposed “patient-based health technology assessments,” key institutions have made progress. PCORI, ICER, and other agencies invite patient comments on HTAs and listen to patients on their advisory panels. Avalere, a healthcare consultancy, published guidance on assessing patient-value. Nonetheless, the methods for including the patient perspective in HTAs are neither well-defined, nor widely adopted.
Implementing patient-centered HTAs is challenging. Doing so demands large budgets for research, modeling, and patient outreach. Tensions among HTA stakeholders, described below, have slowed progress:
Payer vs. Patient Objectives
Technology Assessments were founded in part to control health insurance spending. Is this objective–to keep costs down, or more controversially “to ration care,” at odds with patient objectives? While some economists fear these diverging interests cannot be overcome, patient perspectives may benefit payers. As Bridges and Jones (2007) point out,
“By adopting a patient-based approach, it is quite probable that we end up finding society paying less in aggregate, while getting better outcomes. Paradoxically, we could find ourselves having a more cost-effective outcome than if we were obsessed with cost-effectiveness because we would empower patients and stop giving them care that they neither want nor need.”
Population vs Individuals
Patients long to be heard and seen; they want their unique, individual experiences captured. Health economic evaluation, however, occurs on the population level. HTAs determine the best allocation of resources for a population of insured people on a given health plan or the general public. Guidelines on cost-effectiveness analysis state that weights assigning value to health states should be “based on community preferences rather than those of patients” or providers. Patients value health states differently from the general public. Evidence shows, patients rank problems related to mobility and daily activities as less severe, but consider problems related to pain, discomfort, and anxiety as more severe.
Health economists devoted to patient-centricity must employ methods that acknowledge individual variation in preferences, while uncovering population-level insights. For example, one wheel-chair bound patient with Spinal Muscular Atrophy (SMA) measures health progress by his ability to open the refrigerator door. How can outcomes important to patients be reflected in HTAs? While it’s impossible to consider every patient’s preference, adopting patient-relevant endpoints and surrogate endpoints uncovers what “value” means for patients and caregivers.
Qualitative vs Quantitative
HEOR techniques require statistical prowess. As one patient leader noted, ‘data people’ developing HTAs are often removed from the touchy-feely side of healthcare. In many cases (though not always!) economic modelers are disconnected from the day-to-day emotions of patients and caregivers. An ongoing challenge is bridging quantitative approaches to value assessment with qualitative approaches. ICER’s value framework considers ‘additional benefits’ of therapies, like reduced caregiver burden. New techniques may emerge to bridge this gap. The points of tension in HTAs –payers vs. patients, populations vs individuals, and qualitative vs quantitative– are intricately related. To overcome these barriers, drug manufacturers, patient organizations, technology companies, and payers must collaboratively design HTA methods.
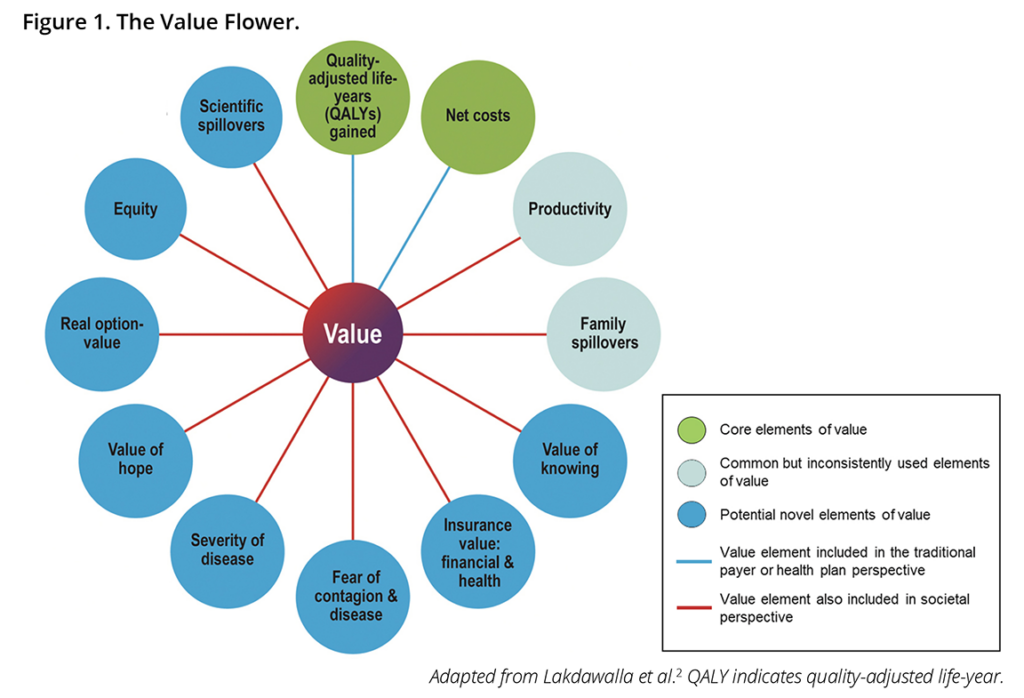
Leaders in HEOR, including the ISPOR president, are calling for patient input in HTA methods as well. The ISPOR Value Flower, which serves to guide HTAs, examines other measures of value, like health equity and the value of hope. Funding agencies and regulatory bodies increasingly require inclusion of the patient voice in research. The Center for Medicare & Medicaid Services (CMS) indicated that it will consider patient experience data in the Inflation Reduction Act (IRA) drug price negotiations. Avalere recommends,
“Developing standardized instruments that capture the patient perspective on treatment value could be an important strategy for manufacturers directly and indirectly impacted by IRA Medicare negotiation.”
PCORI, a government agency founded in 2012, provides funding for patient-centered cost-effectiveness research. The FDA accepts data from drug manufacturers on patient-reported outcomes.. Drug and device companies seeking to guarantee access to their therapies should be ready to adopt methods for patient-centered HTA.
To adopt patient-centered research in HTAs, stakeholders must accept and agree upon guiding principles. Despite controversy surrounding methods of assess value (like QALYS), some practices are well-established. In January, 2024, the National Pharmaceutical Council published useful guidance on patient-centered HTAs. Key points include,
Lookout for emerging guidance on HTAs from the Innovation and Value Institute.
Do you want to further the development of patient-centered HTA methods? Share your ideas with Nested Knowledge. We welcome input from users on how to design AI-research technology to support patient centered research! Chat with Nested Knowledge at ISPOR 2024 in Atlanta by stopping by our booth or by attending the IVI’s networking event! Can’t attend ISPOR? Email Nested Knowledge, book a demo, or share a suggestion on our product board.
Nested Knowledge is a software platform for building living evidence libraries. The research portion of the software ‘AutoLit’ integrates with PubMed, clinicaltrials.gov, openAI, and Scite, allowing users to rapidly retrieve and extract the latest clinical evidence. The evidence portion of the software ‘Synthesis’ displays customizable dashboards and interactive diagrams automatically generated from literature reviews.
Yep, you read that right. We started making software for conducting systematic reviews because we like doing systematic reviews. And we bet you do too.
If you do, check out this featured post and come back often! We post all the time about best practices, new software features, and upcoming collaborations (that you can join!).
Better yet, subscribe to our blog, and get each new post straight to your inbox.

One of the features of Core Smart Tags is Smart Study Type – this refers to our AI system that automatically categorises the study type