
Introducing Core Smart Tags
Introducing Core Smart Tags If you are familiar with Tagging in Nested Knowledge, you know how integral the process of setting up a tagging hierarchy


On January 1st, 2025, a European Union (EU) program will take effect to evaluate the relative benefit and cost-effectiveness of cancer medications and gene therapies. The program, called EUnetHTA 21, will expand to rare disease drugs in 2028. The regulation aims to centralize the Health Technology Assessments (HTAs) across the EU.
In contrast to traditional drug approvals evaluating the safety and efficacy of new therapies, Health Technology Assessments also introduce the questions: “is the drug worth paying for?” and “what other data must be considered?” The scope of HTAs are based on parameters known as PICOs:
HTA regulatory bodies issue guidance that helps payors decide which therapies to cover. Different countries in the EU have their own HTA bodies, which use their own assessment criteria when evaluating a drug. While some HTA bodies focus on how drug coverage impacts payors, others focus on how coverage impacts society. Because of the heterogenous assessments across countries and the need for different strategies for each approach, the process is currently messy and disjointed. There has been no standard data collection framework–until now.
The EUnetHTA regulation attempts to standardize HTAs, reducing redundancy. The key output is a Joint Clinical Assessment (JCA), a report produced by multiple European countries that informs drug pricing and reimbursement. By 2030, the EUnetHTA will apply to all drugs, in vitro diagnostics, and high-risk medical devices in the European market. Participation is mandatory for manufacturers.
The EU’s new Health Technology Assessment method begins within four-part Scoping process:
The consolidated PICOs are agnostic to data availability. Drug manufacturers, therefore, must be ready to conduct literature searches encompassing multiple databases, preprint libraries, and posters.
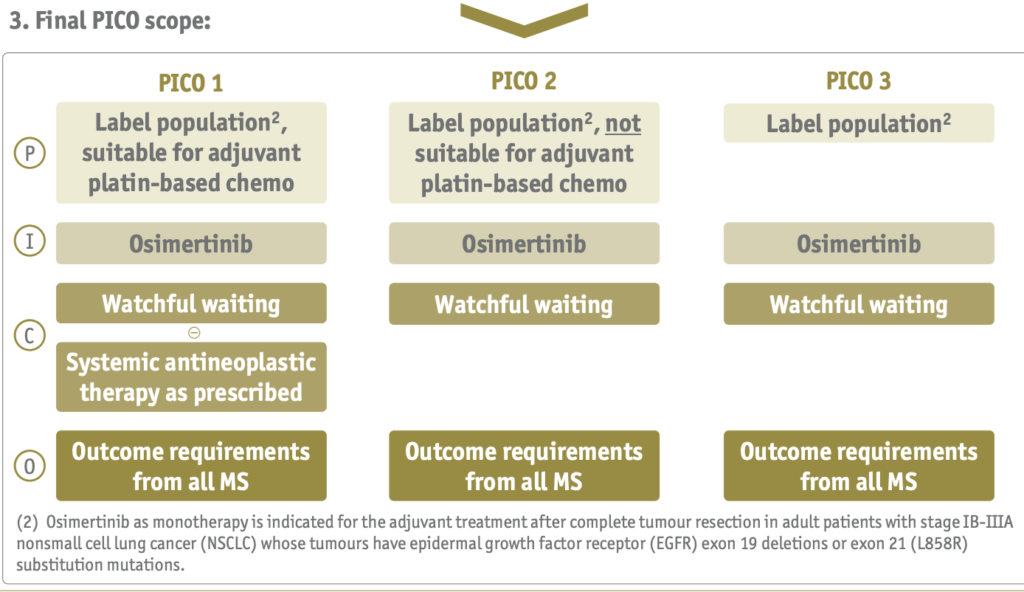
PICOs function as a map of key concepts to seek, guiding drug manufacturers and their consultancies on what data to gather while constructing dossiers. The populations, interventions, comparators, and outcomes selected vary based on the policy needs of the respective EU countries’ health systems. In a poster presented at ISPOR Europe, researchers highlighted the variety in PICO requirements from EU member states. PICO schemes for three cancer drugs were retrieved from national HTA reports for six countries, and then consolidated with the EUnetHTA methods. PICO consolidation resulted in 1-3 PICOS. With 8-10 countries weighing in, the number of PICO schemes and endpoints will be higher. An IQVIA analysis predicted, under the EUnetHTA scoping process, an assessment of Non-Small Cell Lung Cancer will result in 10 PICO schemes and over 280 requested analyses.
In this example, the final PICOs cover multiple populations, including patients suitable for chemotherapy and patients not suitable for chemotherapy.
Despite the regulation’s intention to consolidate evidence demands, multiple PICOs remain. The EUnetHTA estimates an average of 5-6 PICO schemes per assessment!
The key to note here is that PICOs will be submitted by each member country, and the consolidation (or harmonization) of PICOs will then put the onus on manufacturers to gather all review data based on PICOs covered by any member country’s submission. The diversity of PICOs–and the timeline to submission–likely require an a priori evidence generation strategy for collecting and maintaining libraries of relevant evidence.
Once PICOs are chosen, drug manufacturers have only 90 days to complete the Joint Clinical Assessment submission dossiers. Dossiers impact the drug’s price and coverage. Patient access to new therapies depends upon effective dossiers. A strong evidence strategy will anticipate likely PICOs before the final PICO determination. Best practices are to launch an evidence strategy before completing the phase III clinical trial design– as early as four years before drug approval in Europe. This may seem intimidating or premature. However, given the complexity of harmonizing PICOs and the importance of ensuring that the right endpoints and statistical power are available for the eventual dossier, no time is too early!
While the 90-day submission of a review across diverse PICOs may be intimidating, emerging technologies can help structure and perform the required data gathering. Specifically, the use of living evidence libraries, which are rapidly updated as new evidence emerges, positions manufacturers to swiftly adapt to evolving evidence requirements. Here, one solution, a centralized living evidence library software offering by Nested Knowledge, is described in brief.
Transparency, updateability, and comprehensiveness are baked into Nested Knowledge’s living evidence platform. The software was co-developed with ECRI, an Evidence-based Practice Center dedicated to gathering safety and efficacy data. Nested Knowledge’s features prepare HEOR and Market Access Teams for PICO-driven evidence synthesis and literature reviews.
Search. Nested Knowledge integrates with clinical databases like PubMed and ClinicalTrials.gov and allows users to upload existing evidence on hand. You can automatically refresh literature searches daily, weekly, or monthly. Watch a guide on constructing a search based on PICOs.
Screening with PICOs in Mind. When screening study abstracts/full texts for inclusion in your HTA submission dossier, you may use the tool RoboPICO. RoboPICO is an automatic PICO highlighting tool, used to find the most commonly mentioned Populations, Interventions, and Outcomes in the abstracts and titles.
Nest Copy and Protocol Templates. Your team can easily iterate upon old projects, i.e. “nests” by copying the nest and adjusting the scope of included references. With custom evidence filters, you can limit studies to those tagged with a pre-configured concept such as, “Suitable for chemotherapy” or “Quality of Life Outcomes.” Building off previous work is critical for meeting the EUnetHTA submission deadlines. Organizations may also design and reuse templates or borrow HTA templates from Nested Knowledge.
Save Time with AI. Completing 5-6 literature reviews (1 for each PICO) in 90 days may seem daunting. Nested Knowledge offers a friendly Robot Reviewer that will make screening decisions to be ultimately adjudicated by a human-in-the-loop. Our AI Smart Tags extract data from underlying literature using OpenAI’s large language models.
Instant Visuals. When you gather evidence with Nested Knowledge, we instantly create plots, tables, dashboards, and embeddable data visuals. Our interactive evidence has been used by the FDA. Avoid the costs of commissioning Forest Plots or growing frustrated with Excel charts!

The new European HTA Regulation is designed to eliminate waste and reduce redundant work by centralizing HTAs into one Joint Clinical Assessment. JCAs will be consumed by EU countries to inform their reimbursement decisions. While the regulation’s aspirations may resolve heterogeneity of countries’ requirements, the scope of assessments is large and difficult to undertake in the time allotted; manufacturers must prepare early and adapt swiftly. Building and maintaining living evidence libraries in Nested Knowledge is an effective way to compile data and ensure patient access and appropriate pricing to innovative therapies.
Yep, you read that right. We started making software for conducting systematic reviews because we like doing systematic reviews. And we bet you do too.
If you do, check out this featured post and come back often! We post all the time about best practices, new software features, and upcoming collaborations (that you can join!).
Better yet, subscribe to our blog, and get each new post straight to your inbox.

Introducing Core Smart Tags If you are familiar with Tagging in Nested Knowledge, you know how integral the process of setting up a tagging hierarchy